Список публикаций Лаборатории синтеза антибиотиков, преодолевающих резистентность (САПР)
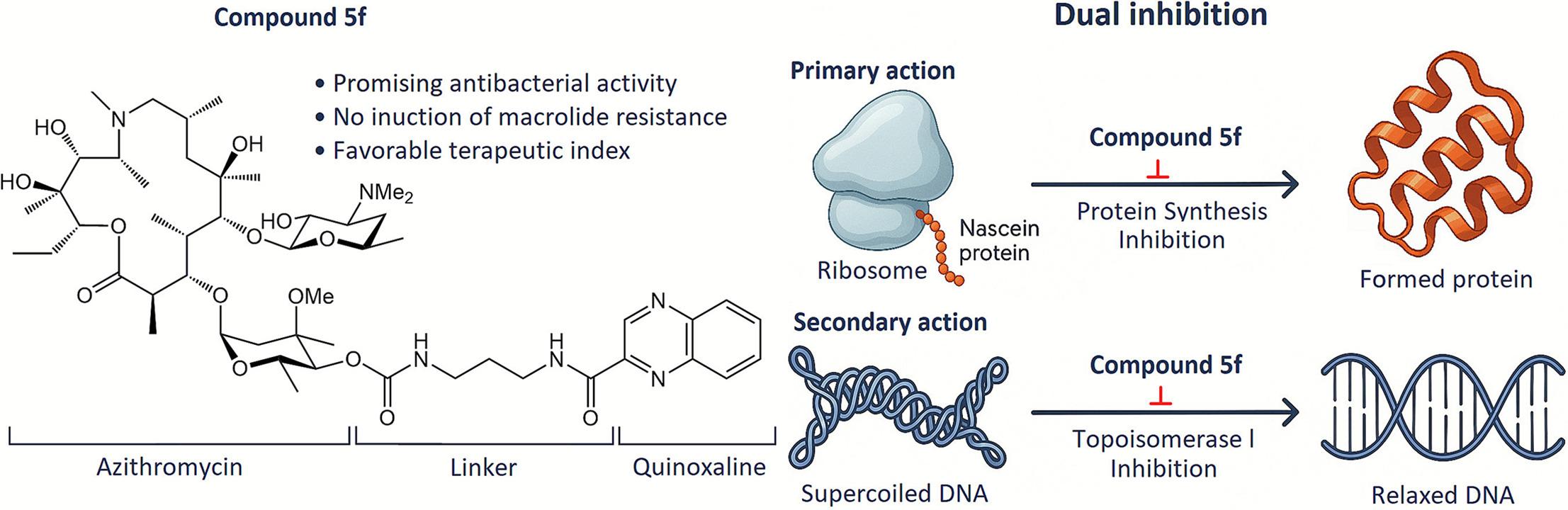
M.M. Martynov, I.A. Volynkina, N.E. Grammatikova, M.A. Kryakvin, N.D. Dadaev, A.O. Karakchieva, O.A. Dontsova, G.V. Zatonsky, A.S. Tikhomirov, A.E. Shchekotikhin. 4″-Modified azithromycin derivatives bearing nitrogen-containing heterocycles with dual inhibitory activity. Bioorganic Chemistry 2026, 169, 109396. doi:10.1016/j.bioorg.2025.109396.
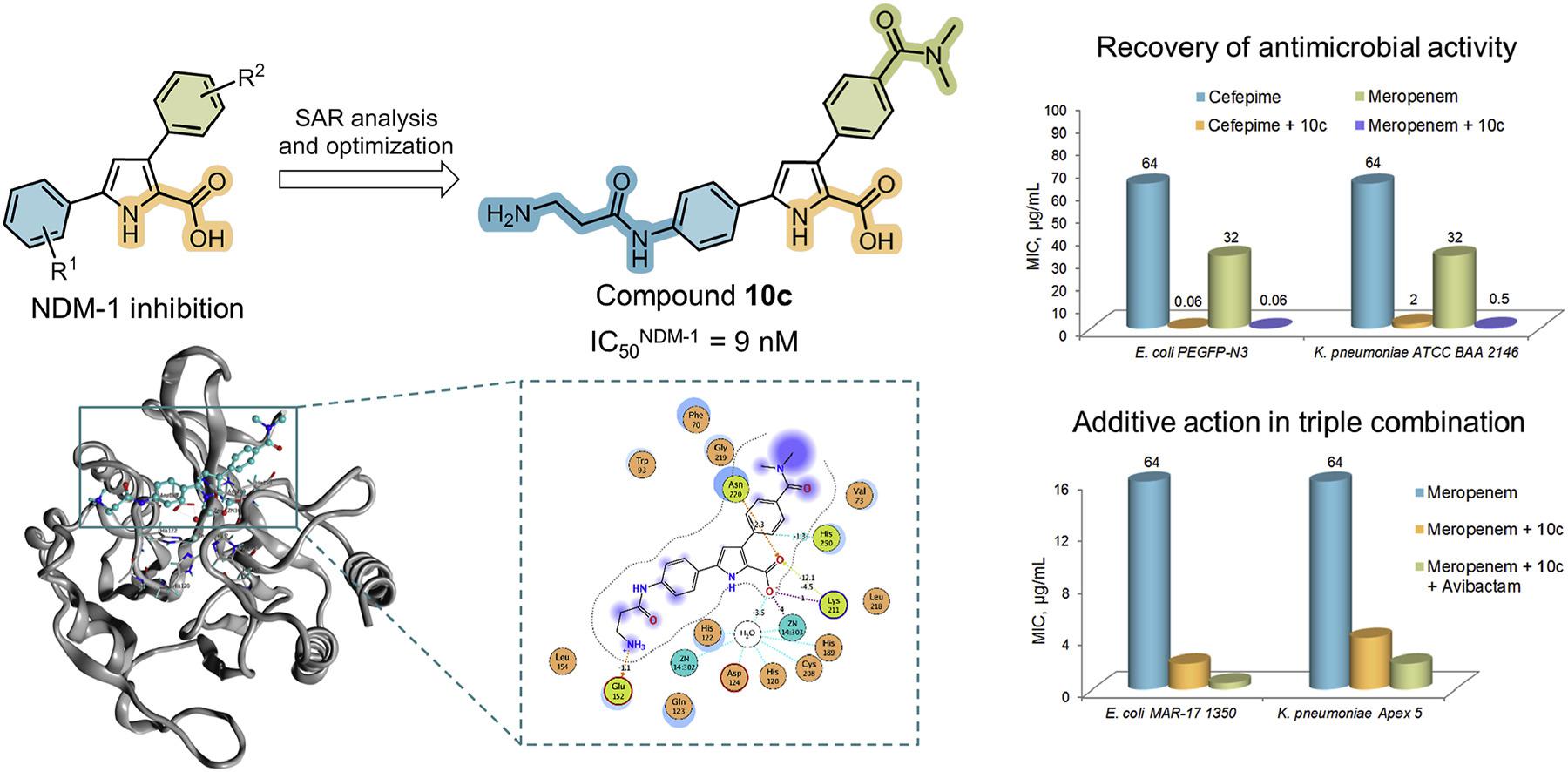
T.S. Shkuratova, K.V. Rychev, V.G. Grigorenko, I.P. Andreeva, N.E. Grammatikova, A.S. Tikhomirov, A.M. Egorov, A.E. Shchekotikhin. Rational Design of 1H-pyrrole-2-carboxylic Acid Inhibitors of NDM-1 Metallo-β-lactamase Restoring β-Lactam Efficacy Against Resistant Enterobacterales. European Journal of Medicinal Chemistry 2026, 302(3), 118376; https://doi.org/10.1016/j.ejmech.2025.118376
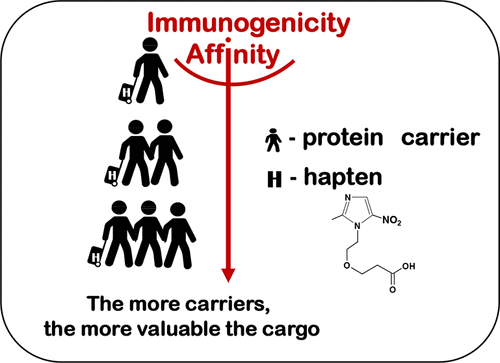
M.A. Burkin, V.A. Litvinova, A.S. Tikhomirov, E.Y. Katarzhnova, I.A. Galvidis. Effect of Carrier Protein Size on Hapten Immunogenicity and Antibody Affinity in Mice. Bioconjugate Chemistry 2026, 37, 1, 216–224 https://doi.org/10.1021/acs.bioconjchem.5c00606.
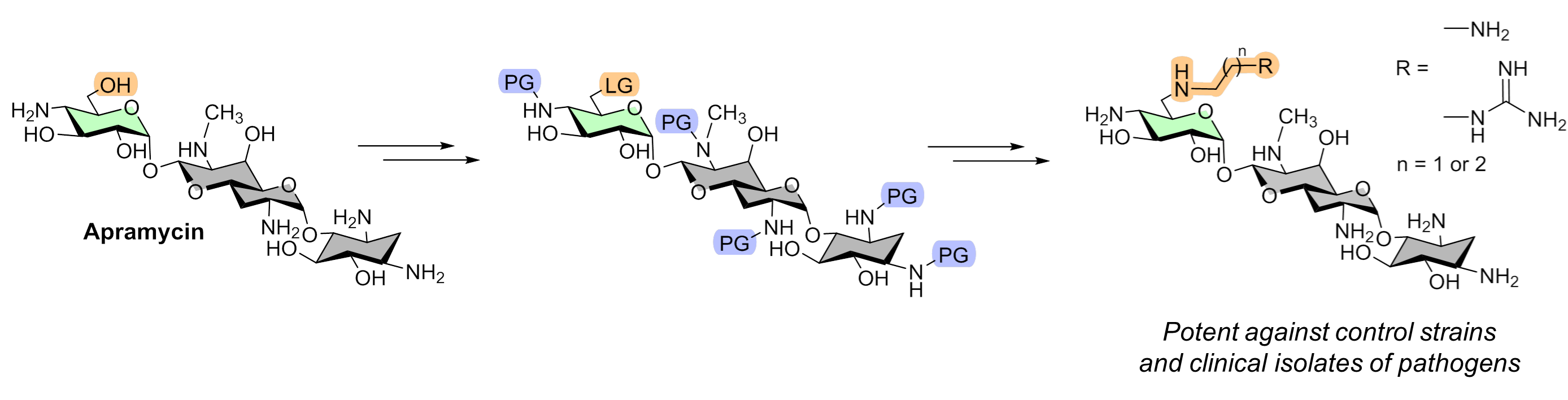
K.S. Shapovalova, G.V. Zatonsky, E.A. Razumova, N.D. Dagaev, D.A. Lukianov, N.E. Grammatikova, A.S. Tikhomirov, A.E. Shchekotikhin. Design, Synthesis, and Biological Evaluation of 6″-Modified Apramycin Derivatives to Overcome Aminoglycoside Resistance. Pharmaceutics 2025, 17, 1583; https://doi.org/10.3390/pharmaceutics17121583
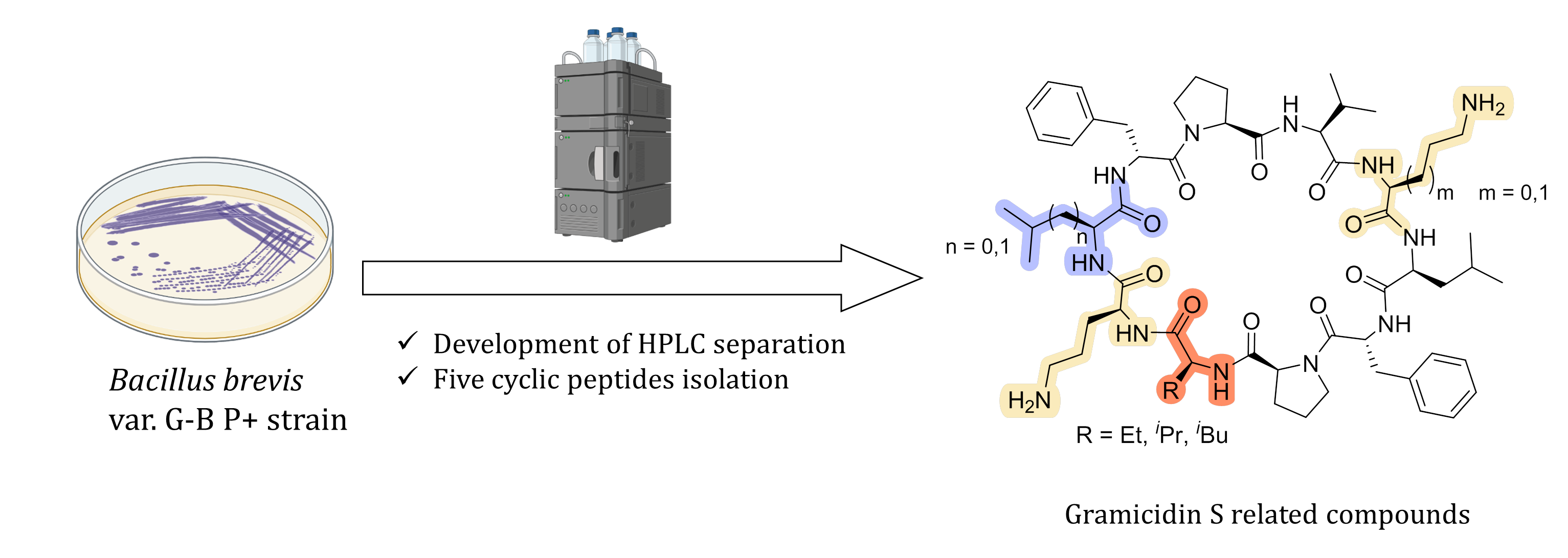
A.S. Tikhomirov, S.E. Solov’eva, T.S. Shkuratova, N.M. Malyutina, D.V. Andreeva, G.V. Zatonsky, N.E. Grammatikova, A.E. Shchekotikhin. Isolation of Cyclic Decapeptide Antibiotics Related to Gramicidin S. Natural Product Research 2025, 1–5; https://doi.org/10.1080/14786419.2025.2568953
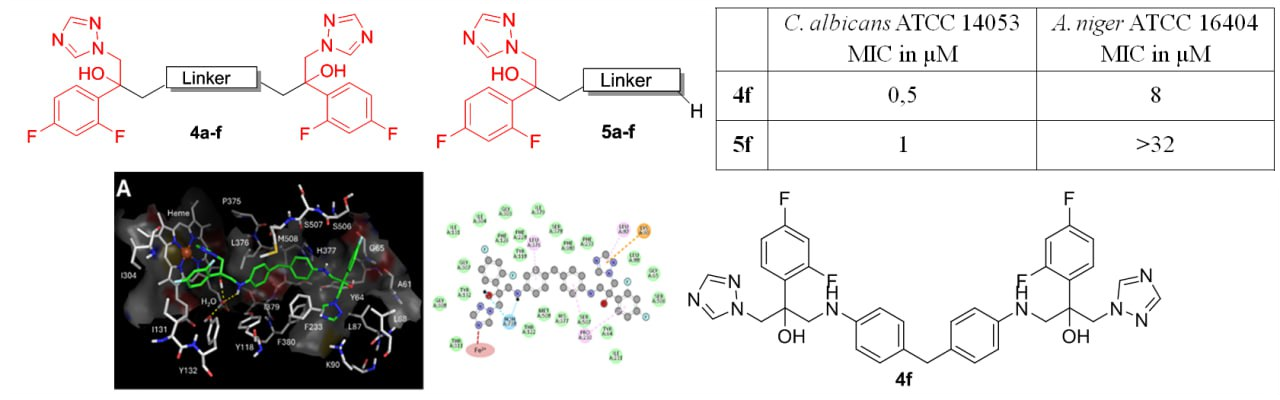
S.N. Lavrenov, A.Yu. Simonov, A.A. Panov, S.E. Solovieva, A.B. Mantsyzov, A.V. Churakov, A.S. Trenin, I.B. Levshin. Azole Homodimers as Promising Antifungal Agents: Synthesis, Biological Activity Evaluation and Molecular Modeling. Current Pharmaceutical Design 2025, 32(37), 8479–8493; https://doi.org/10.2174/0109298673351661241219053546
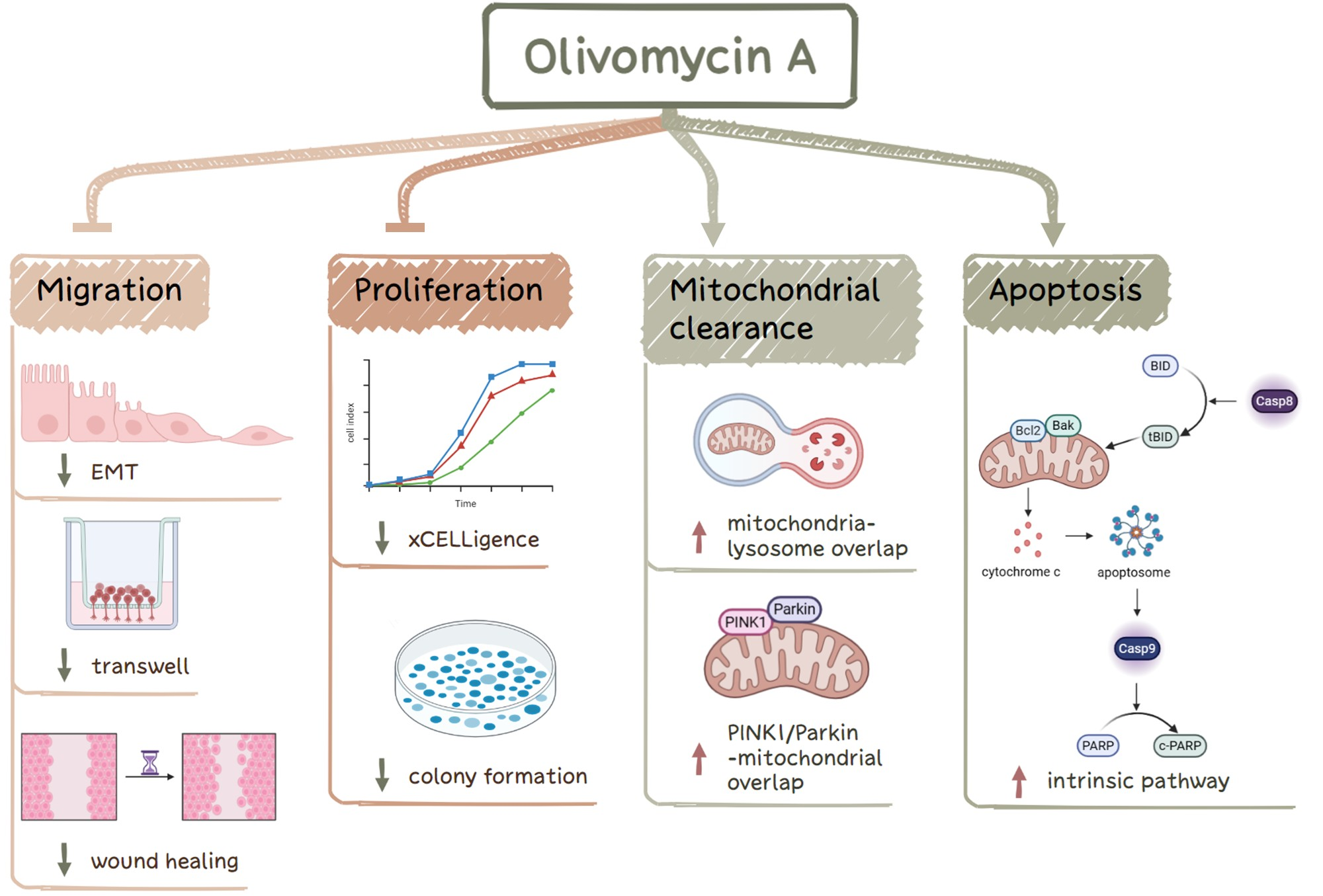
C.-Y. Hsieh, Y.-F. Liou, Y.-T. Shih, A.S. Tikhomirov, A.E. Shchekotikhin, P.J. Chueh. Olivomycin A Targets Epithelial–Mesenchymal Transition, Apoptosis, and Mitochondrial Quality Control in Renal Cancer Cells. Antioxidants 2025, 14, 1348; https://doi.org/10.3390/antiox14111348
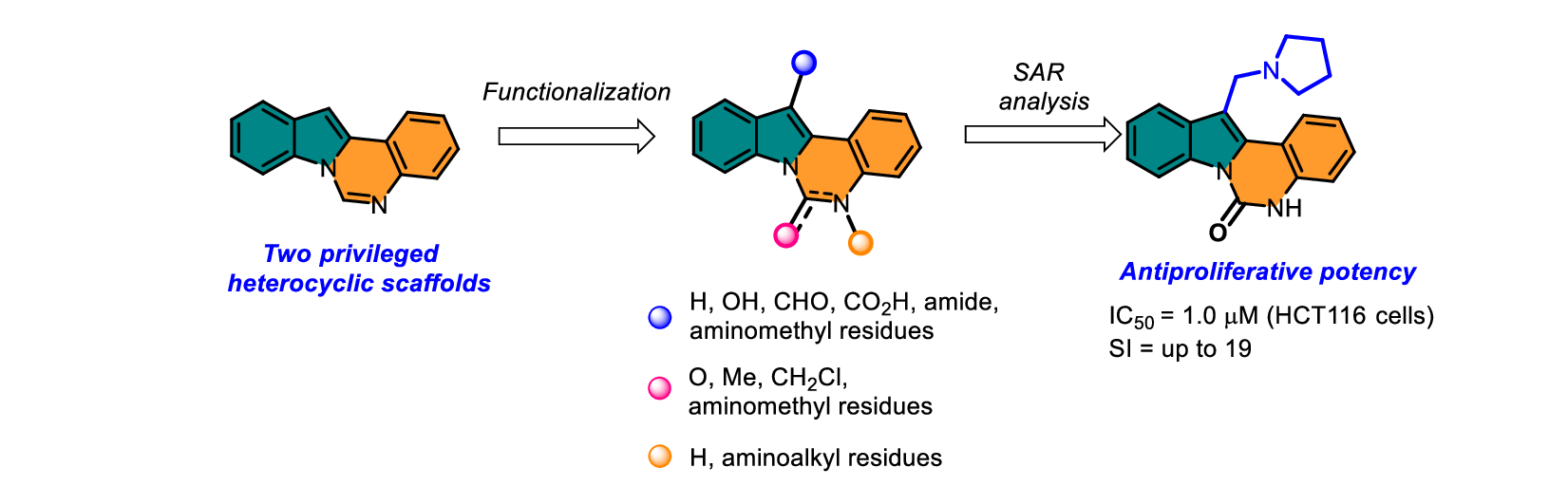
D.V. Khabarov, V.A. Litvinova, L.G. Dezhenkova, D.N. Kaluzhny, A.S. Tikhomirov, AE Shchekotikhin. Discovery of cytotoxic indolo[1, 2-c]quinazoline derivatives through scaffold-based design. Beilstein Journal of Organic Chemistry 2025, 21(1), 2062-2071; https://doi.org/10.3762/bjoc.21.161.
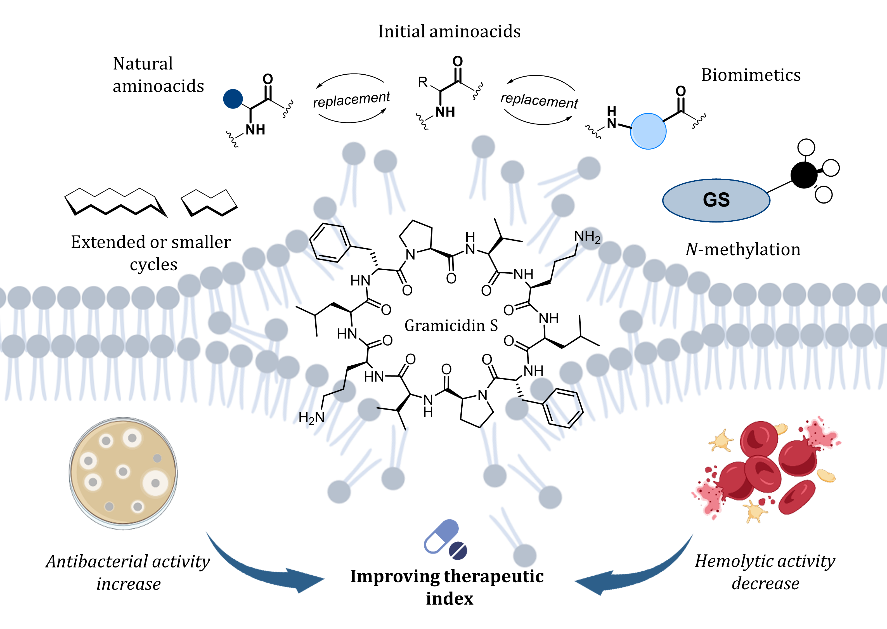
T.S. Shkuratova, D.V. Andreeva, A.S. Tikhomirov, A.E. Shchekotikhin. Analogues of gramicidin S: A promising direction for future antibacterial drug development? European Journal of Medicinal Chemistry, 2025, 297, 117955; https://doi.org/10.1016/j.ejmech.2025.117955.
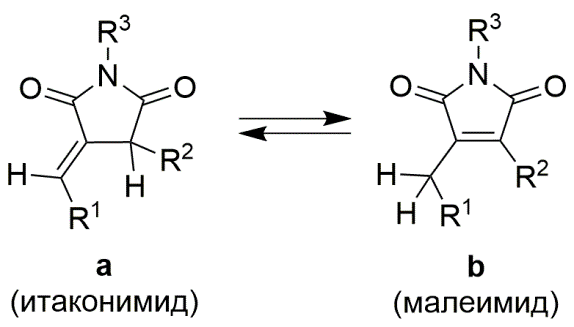
Панов А.А. Квантовохимическое исследование энергий изомерных производных малеимида и итаконимида. Журнал физической химии, 2025, Т. 99, № 4, 605-610; https://doi.org/10.31857/S0044453725040098 [Panov A.A. Quantum chemical study of the energies of isomeric maleimide and itaconimide derivatives. Russian Journal of Physical Chemistry, 2025, Т. 99, № 3, 522-527; https://doi.org/10.31857/S0044453725040098]
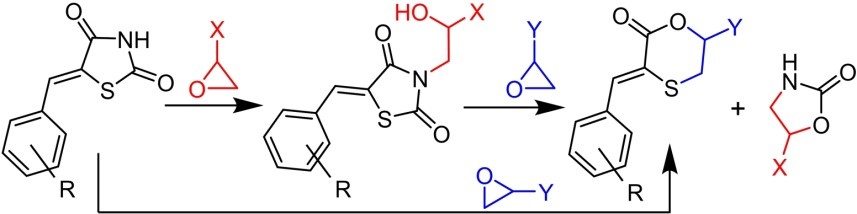
Simonov A.Yu., Panov A.A., Churakov A.V., Polshakov V.I., Levshin I.B. Oxirane-driven cascade transformations of 5-arylidene-thiazolidine-2,4-diones. Asian Journal of Organic Chemistry, 2025, Т. 14, № 3; https://doi.org/10.1002/ajoc.202400645
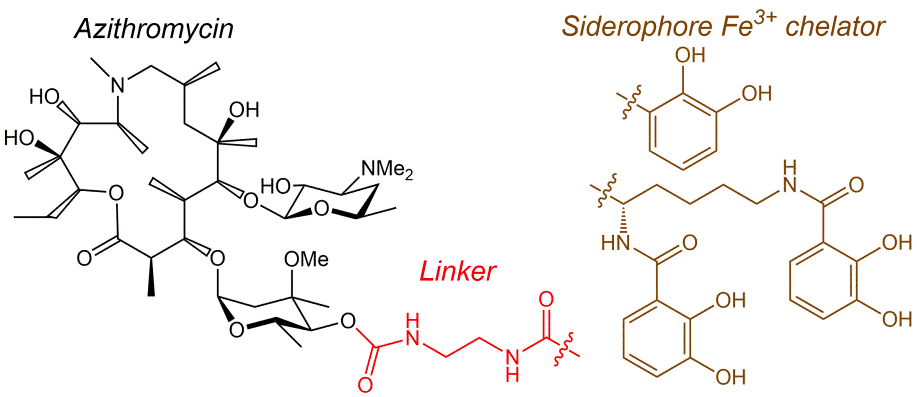
M.M. Martynov, N.E. Grammatikova, G.V. Zatonsky, S.E. Solovieva, A.S. Tikhomirov, E.N. Bychkova, A.E. Shchekotikhin, Synthesis and Estimation of the Antibacterial Potency of a Series of Azithromycin-Siderophore Conjugates. Current Organic Chemistry, 2025; https://doi.org/10.2174/0113852728404096250908065829
K.S. Shapovalova, G.V. Zatonsky, E.A. Razumova, D.A. Ipatova, D.A. Lukianov, P.V. Sergiev, N.E. Grammatikova, A.S. Tikhomirov, A.E. Shchekotikhin. Synthesis and antibacterial activity of new 6″-modified tobramycin derivatives, Antibiotics 2024, 13(12), 1191; https://doi.org/10.3390/antibiotics13121191

T.S. Shkuratova, V.G. Grigorenko, I.P. Andreeva, V.A. Litvinova, N.E. Grammatikova, A.S. Tikhomirov, A.M. Egorov, A.E. Shchekotikhin. New derivatives of dipicolinic acid as metallo-β-lactamase NDM-1 inhibitors. Medicinal Chemistry Research, 2024, https://doi.org/10.1007/s00044-024-03330-z

M.A. Burkin, A.S. Tikhomirov, Y.A. Surovoy, I.A. Galvidis. Hapten synthesis, antibody generation, and immunoassay development for linezolid therapeutic monitoring, Analytical Chemistry 2024, 96, 44, 17859–17867, https://doi.org/10.1021/acs.analchem.4c04537

O. Omelchuk, E. Bychkova, S. Efimova, N. Grammatikova, G. Zatonsky, L. Dezhenkova, S. Solovieva, O. Ostroumova, A. Tevyashova, A. Shchekotikhin, Mono-N-alkylation of Amphotericin B and Nystatin A1 and Its Amides: Effect on the In Vitro Activity, Cytotoxicity and Permeabilization of Model Membranes, Antibiotics 2024, 13(12), 1177, https://doi.org/10.3390/antibiotics13121177

V.A. Litvinova, V.B. Tsvetkov, Y.L. Volodina, L.G. Dezhenkova, A.A. Markova, M.T. Nguyen, A.S. Tikhomirov, A.E. Shchekotikhin, Naphthoindole-2-carboxamides as a lipophilic chemotype of hetarene-anthraquinones potent against P-gp resistant tumor cells, European Journal of Medicinal Chemistry, 2025, 281, 117013, https://doi.org/10.1016/j.ejmech.2024.117013

I.B. Levshin, A.Y. Simonov, A.A. Panov, N.E. Grammatikova, A.I. Alexandrov, E.S.M.O. Ghazy, V.A. Ivlev, M.O. Agaphonov, A.B. Mantsyzov, V.I. Polshakov, Synthesis and Biological Evaluation of a Series of New Hybrid Amide Derivatives of Triazole and Thiazolidine-2,4-dione, Pharmaceuticals, 2024, 17, 723; https://doi.org/10.3390
/ph17060723
A.A. Panov, New Methods of Synthesis of Fused Maleimides, Russian Journal of Organic Chemistry, 2024, 60, 553–567; https://doi.org/10.1134/S1070428024040018

D.V. Andreeva, A.S. Tikhomirov, A.E. Shchekotikhin, Synthesis and antiproliferative activity of thiazole-fused anthraquinones, Organic & Biomolecular Chemistry, 2024, in press; https://doi.org/10.1039/D4OB01284D

V.N. Charushin, E.V. Verbitskiy, O.N. Chupakhin, D.V. Vorobyeva, P.S. Gribanov, S.N. Osipov, A.V. Ivanov, S.V. Martynovskaya, E.F. Sagitova, V.D. Dyachenko, I.V. Dyachenko, S.G. Krivokolysko, V.V. Dotsenko, A.V. Aksenov, D.A. Aksenov, N.A. Aksenov, A.A. Larin, L.L. Fershtat, V.M. Muzalevskiy, V.G. Nenajdenko, A.V. Gulevskaya, A.F. Pozharskii, E.A. Filatova, K.V. Belyaeva, B.A. Trofimov, I.A. Balova, N.A. Danilkina, A.I. Govdi, A.S. Tikhomirov, A.E. Shchekotikhin, M.S. Novikov, N.V. Rostovskii, A.F. Khlebnikov, Yu.N. Klimochkin, M.V. Leonova, I.M. Tkachenko, V.A. Mamedov, V.L. Mamedova, N.A. Zhukova, V.E. Semenov, O.G. Sinyashin, O.V. Borshchev, Yu.N. Luponosov, S.A. Ponomarenko, A.S. Fisyuk, A.S. Kostyuchenko, V.G. Ilkin, T.V. Beryozkina, V.A. Bakulev, A.S. Gazizov, A.A. Zagidullin, A.A. Karasik, M.E. Kukushkin, E.K. Beloglazkina, N.E. Golantsov, A.A. Festa, L.G. Voskresensky, V.S. Moshkin, E.M. Buev, V.Ya. Sosnovskikh, I.A. Mironova, P.S. Postnikov, V.V. Zhdankin, M.S. Yusubov, I.A. Yaremenko, V.A. Vil', I.B. Krylov, A.O. Terent'ev, Yu.G. Gorbunova, A.G. Martynov, A.Yu. Tsivadze, P.A. Stuzhin, S.S. Ivanova, O.I. Koifman, O.N. Burov, M.E. Kletskii, S.V. Kurbatov, O.I. Yarovaya, K.P. Volcho, N.F. Salakhutdinov, M.A. Panova, Ya.V. Burgart, V.I. Saloutin, A.R. Sitdikova, E.S. Shchegravina, A.Yu. Fedorov, The chemistry of heterocycles in the 21st century, Russian Chemical Reviews, 2024, 93 (7), RCR5125; https://doi.org/10.59761/RCR5125

D.V. Andreeva, T.S. Vedekhina, A.S. Gostev, L.G. Dezhenkova, Y.L. Volodina, A.A. Markova, M.T. Nguyen, O.M. Ivanova, V.A. Dolgusheva, A.M. Varizhuk, A.S. Tikhomirov, A.E. Shchekotikhin, Thiadiazole-, selenadiazole- and triazole-fused anthraquinones as G-quadruplex targeting anticancer compounds, European Journal of Medicinal Chemistry, 2024, 268, 116222; https://doi.org/10.1016/j.ejmech.2024.116222

I.A. Volynkina, E.N. Bychkova, A.O. Karakchieva, A.S. Tikhomirov, G.V. Zatonsky, S.E. Solovieva, M.M. Martynov, N.E. Grammatikova, A.G. Tereshchenkov, A. Paleskava, A.L. Konevega, P.V. Sergiev, O.A. Dontsova, I.A. Osterman, A.E. Shchekotikhin, A.N. Tevyashova, Hybrid Molecules of Azithromycin with Chloramphenicol and Metronidazole: Synthesis and Study of Antibacterial Properties, Pharmaceuticals, 2024, 17 (2), 187; https://doi.org/10.3390/ph17020187

V.A.Litvinova, A.S.Tikhomirov, A.E.Shchekotikhin, Methods for functionalization of anthraquinones, Russian Chemical Reviews, 2024, 93, RCR5141, DOI: https://doi.org/10.59761/RCR5141

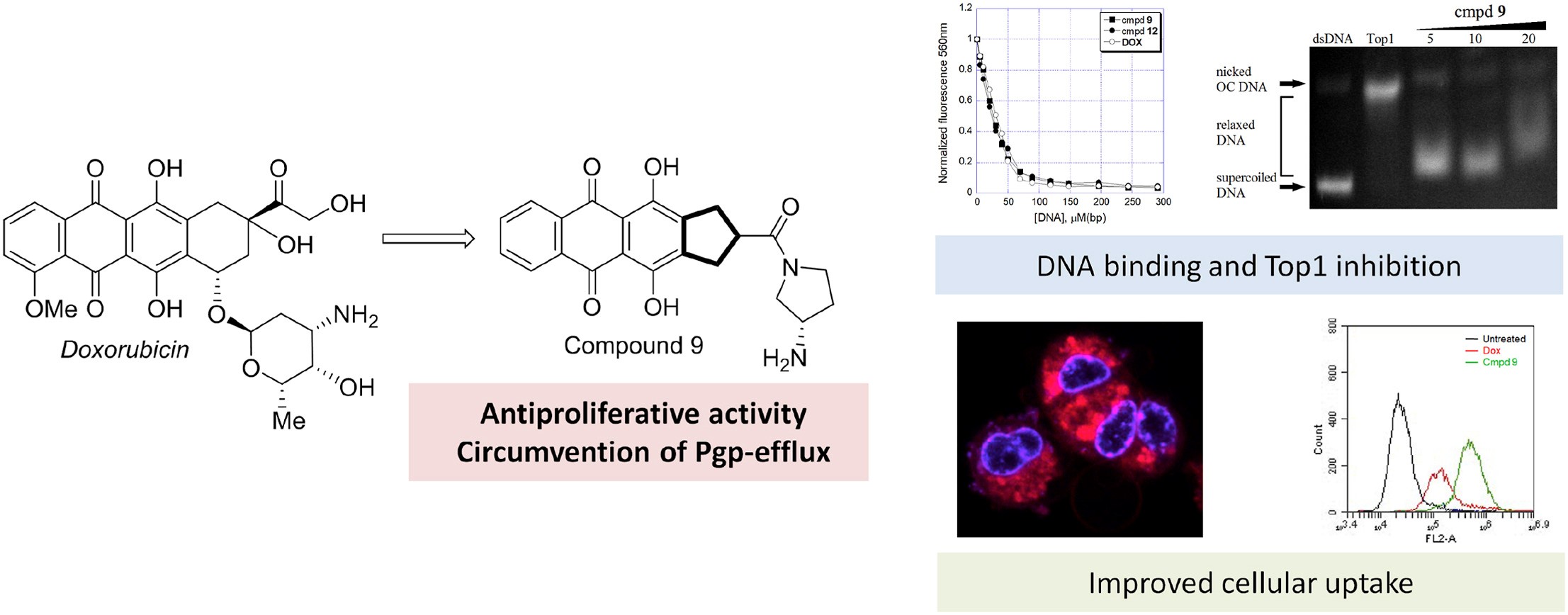
- A.S. Tikhomirov, Y.B. Sinkevich, L.G. Dezhenkova, D.N. Kaluzhny, N.S. Ilyinsky, V.I. Borshchevskiy, D. Schols, A.E. Shchekotikhin, Synthesis and antitumor activity of cyclopentane-fused anthraquinone derivatives, European Journal of Medicinal Chemistry, 2024, 265, 116103, https://doi.org/10.1016/j.ejmech.2023.116103
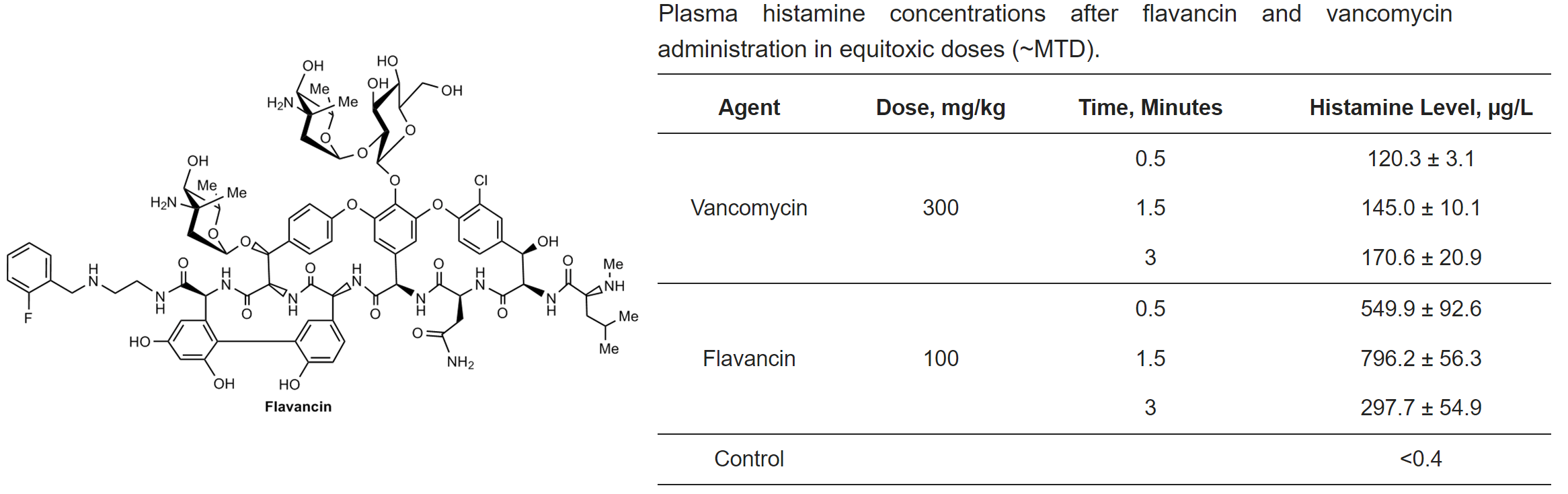
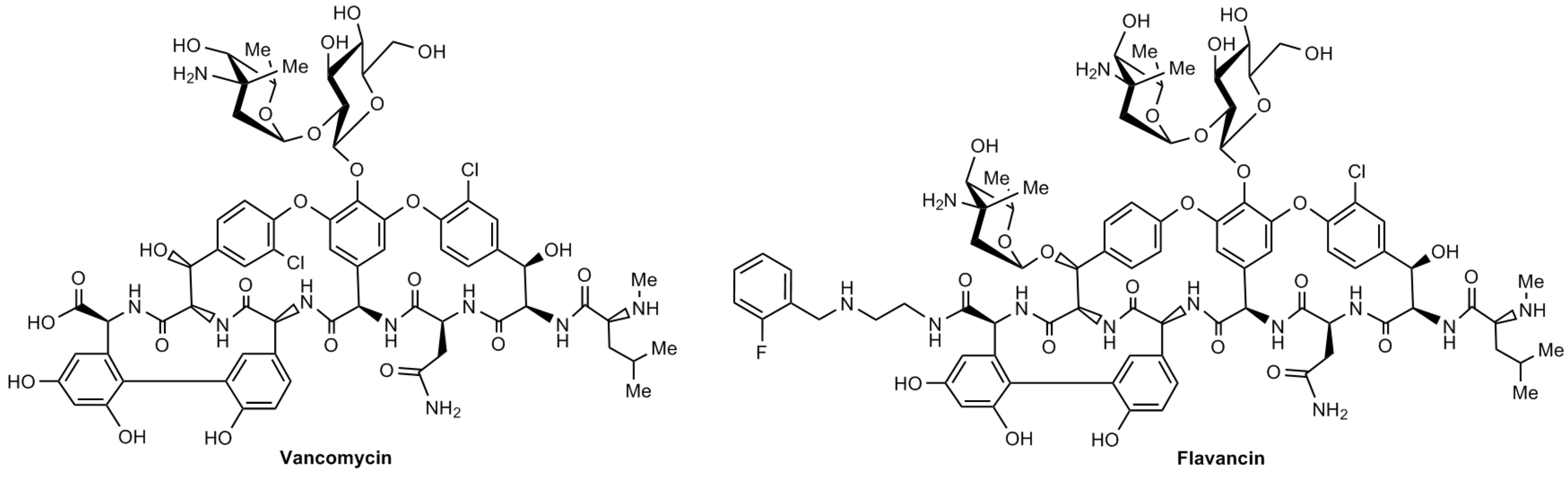
M.I. Treshchalin, V.A. Polozkova, E.I. Moiseenko, A.E. Shchekotikhin, S.A. Dovzhenko, M.B. Kobrin, E.R. Pereverzeva, Experimental Evaluation of the Hypersensitivity Reactions of a New Glycopeptide Antibiotic Flavancin in Animal Models, Pharmaceuticals, 2023, 16(11), 1569; https://doi.org/10.3390/ph16111569
V.A. Litvinova, A.S. Gostev, A.S. Tikhomirov, A.E. Shchekotikhin, New heteroarene-fused anthraquinones: Synthesis and PyBOP-mediated amination, Tetrahedron, 2023, 149, 4, 133722, https://doi.org/https://doi.org/10.1016/j.tet.2023.133722
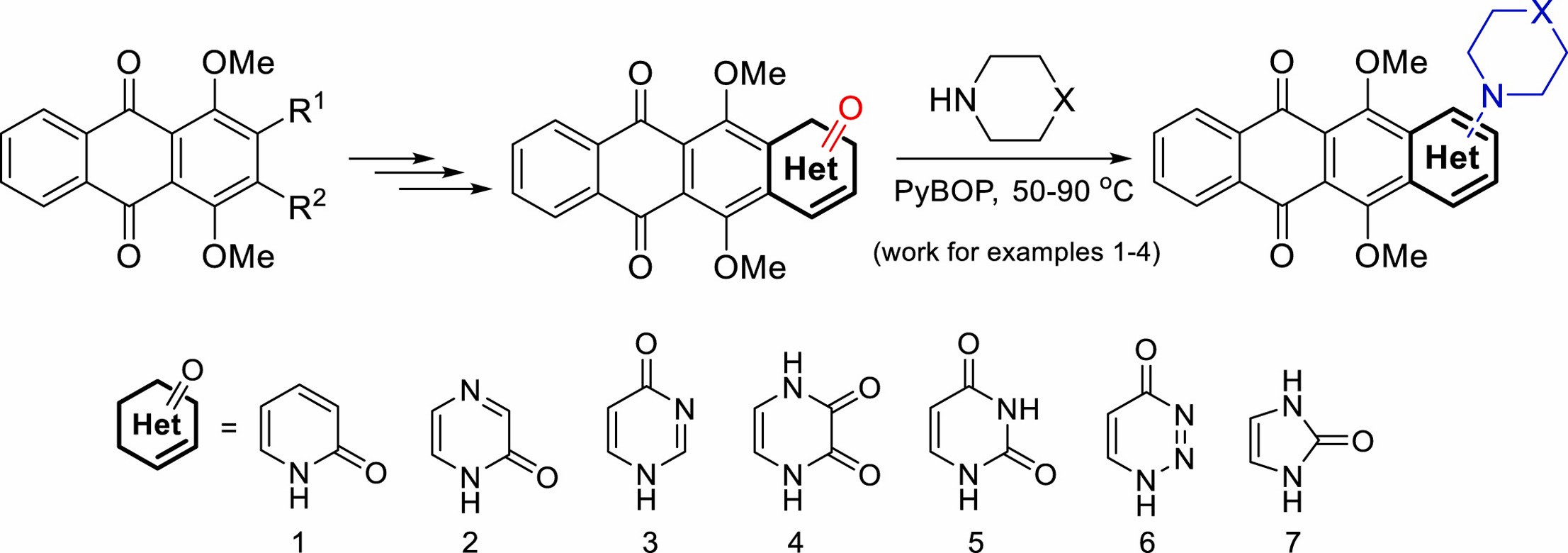
A. Islam, X.C. Chen, C.-W. Weng, C.-Y. Chen, C.-W. Wang, M.-K. Chen, A.S. Tikhomirov, A.E. Shchekotikhin, P.J. Chueh, Water-soluble 4-(dimethylaminomethyl)heliomycin exerts greater antitumor effects than parental heliomycin by targeting the tNOX-SIRT1 axis and apoptosis in oral cancer cells, eLife, 2023, https://doi.org/10.7554/eLife.87873.1
В.А. Литвинова, А.С. Тихомиров, Методы синтеза 1,3-оксазолидин-2-онов, Методы синтеза 1,3-оксазолидин-2-онов, Химия гетероциклических соединений, 2023, 59(11/12), 733-735
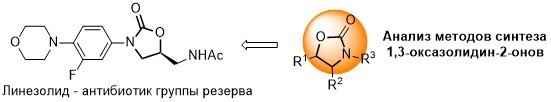
K. Shapovalova, G. Zatonsky, N. Grammatikova, I. Osterman, E. Razumova, A. Shchekotikhin, A. Tevyashova, Synthesis of 6”-Modified Kanamycin A Derivatives and Evaluation of Their Antibacterial Properties. Pharmaceutics, 2023, 15, 1177. https://doi.org/10.3390/pharmaceutics15041177
A.A. Panov, Quantum-Chemical Study of Keto-Enol Equilibrium and Global Electrophilicity of Hydroxymaleimide Derivatives. Doklady Physical Chemistry, 2023, 508, 28–32, https://doi.org/10.1134/S001250162360002X
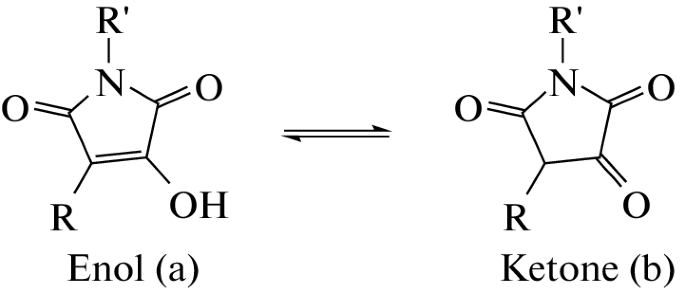
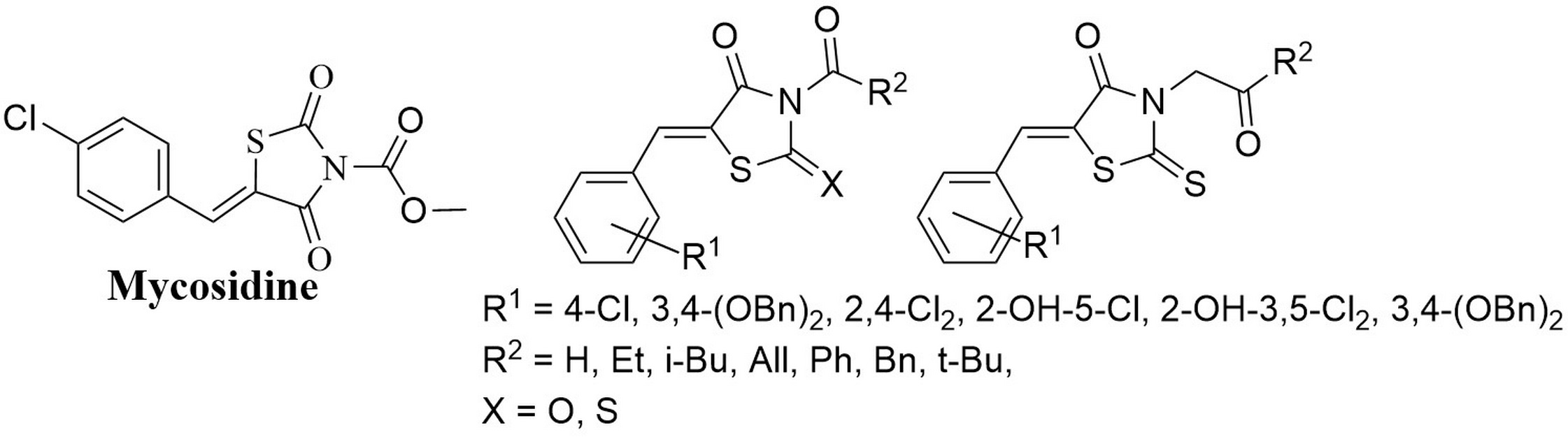
Levshin I.B., Simonov A.Y., Lavrenov S.N., Panov A.A., Grammatikova N.E., Alexandrov A.I., Ghazy E.S.M.O., Savin N.A., Gorelkin P.V., Erofeev A.S., Polshakov V.I. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals, 2022, 15, 5, 563-586. DOI: 10.3390/ph15050563
Treshchalin M.I., Polozkova V.A., Moiseenko E.I., Treshalina H.M., Shchekotikhin A.E., Pereverzeva E.R. Evaluation of Toxic Properties of New Glycopeptide Flavancin on Rats. Pharmaceuticals, 2022, 15, 661. DOI: 10.3390/ph15060661
- A.N. Tevyashova, S.S. Efimova, A.I. Alexandrov, E.S.M.O. Ghazy, E.N. Bychkova, S.E. Solovieva, G.B. Zatonsky, N.E. Grammatikova, L.G. Dezhenkova, E.R. Pereverzeva, E.B. Isakova, O.S. Ostroumova, O.A. Omelchuk, V.V. Muravieva, M.M. Krotova, T.V. Priputnevich, A.E. Shchekotikhin, Semisynthetic Amides of Polyene Antibiotic Natamycin, ACS Infectious Diseases 2022, 2022, https://doi.org/10.1021/acsinfecdis.2c00237
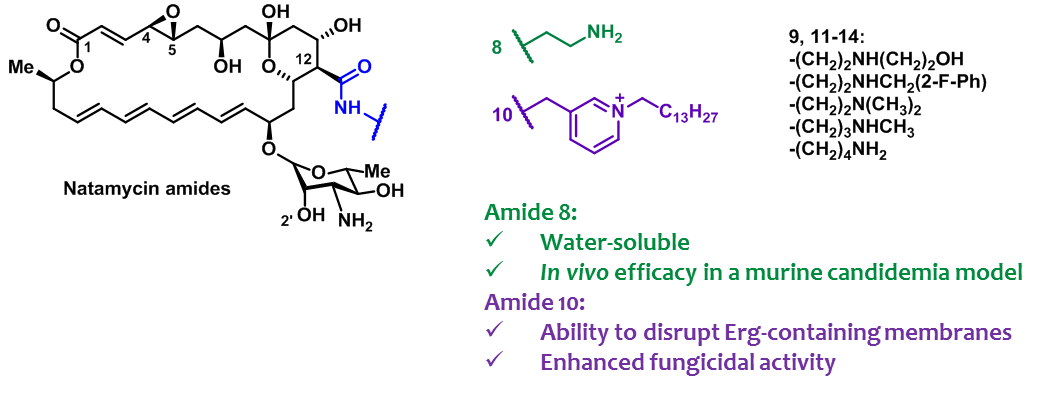
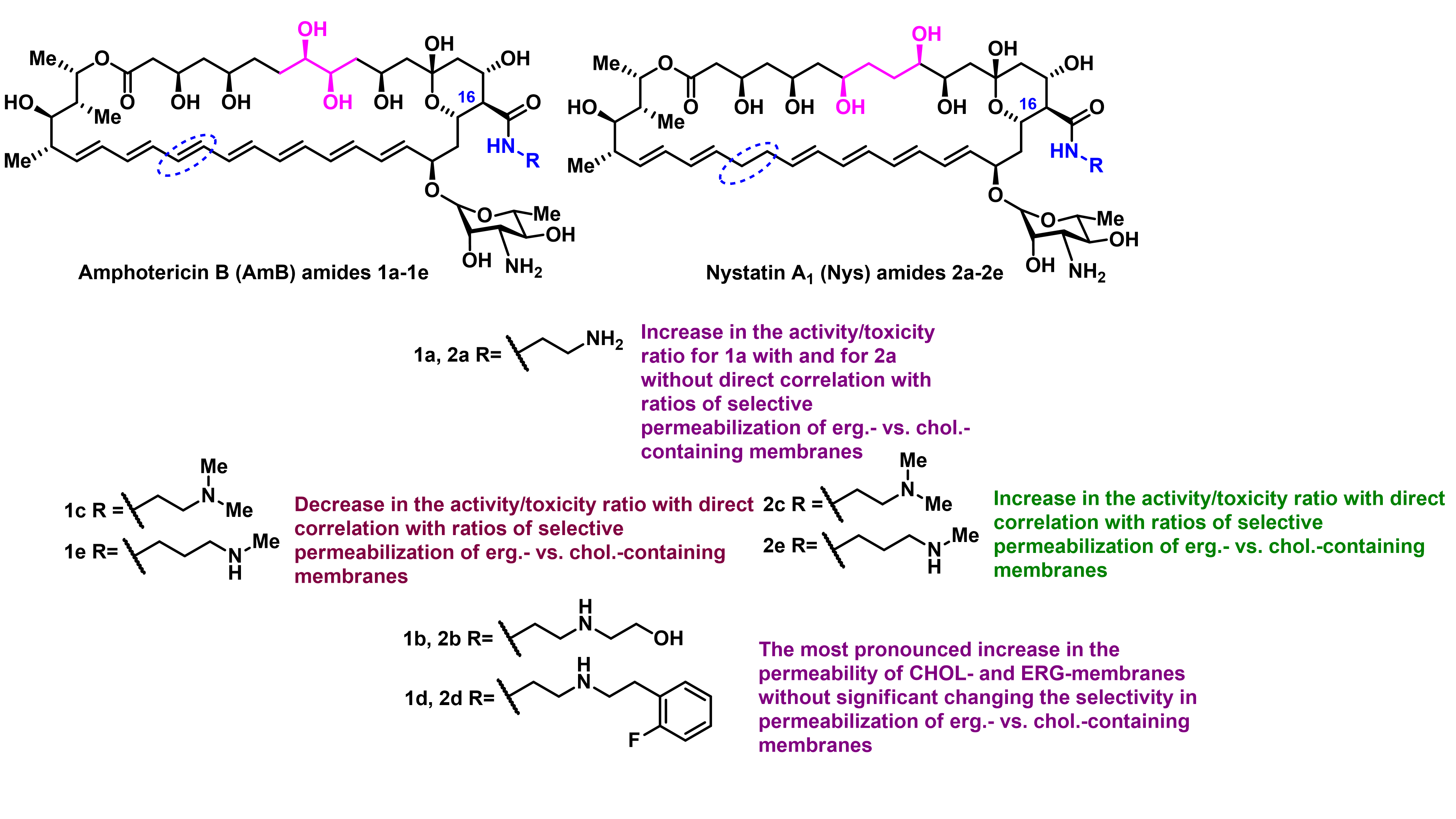
A. Tevyashova, S. Efimova, A. Alexandrov, O. Omelchuk, E. Ghazy, E. Bychkova, G. Zatonsky, N. Grammatikova, L. Dezhenkova, S. Solovieva, O. Ostroumova, A. Shchekotikhin, Semisynthetic Amides of Amphotericin B and Nystatin A1: A Comparative Study of In Vitro Activity/Toxicity Ratio in Relation to Selectivity to Ergosterol Membranes, Antibiotics 2023, 12(1), 151; https://doi.org/10.3390/antibiotics12010151